To Build a Cure
Posted on Jan. 14, 2020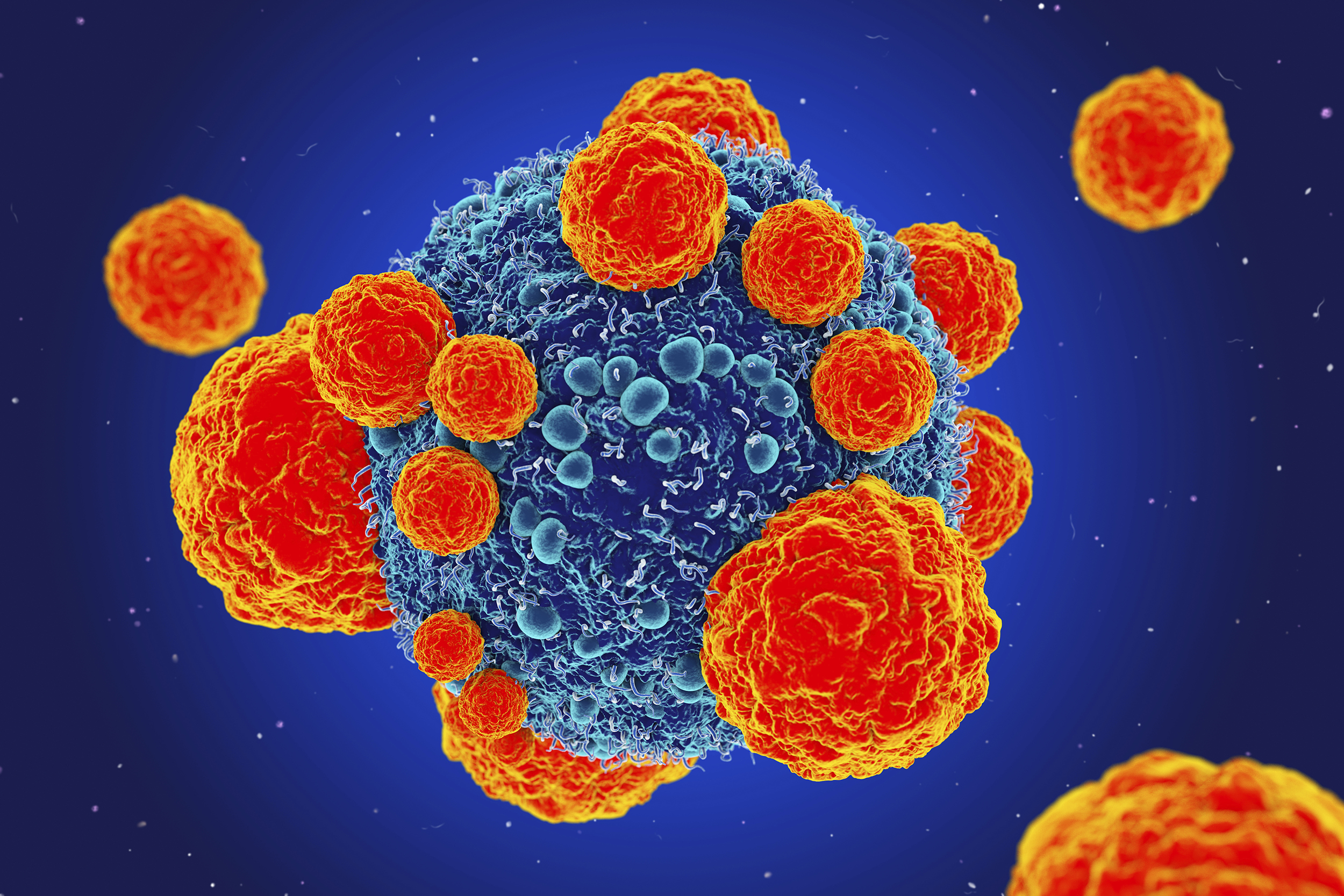
CAR-T is still largely experimental — a futuristic, staggering, frustrating therapy that sometimes works and sometimes does not. (Roger Harris/Science Photo Library)
They’re the most promising cancer assassins to come along in your lifetime, but their mission is far from complete. Where others shied away, UNC rolled out the red carpet for CAR-T therapy’s custom fighter cells.
By Beth McNichol ’95
Sabrina Shelton was at work in 2008 when her mother rushed through the door, gathering her for the first of too many battles ahead.
“We have to go, now,” her mother said. “They have a room waiting for you.”
As they drove to the hospital in Lynchburg, Va., Shelton, who had graduated from Radford University with a psychology degree four months earlier, digested the news that would upend the 22-year-old’s life. The results of Shelton’s blood test at a clinic the day before had just come back, and the numbers were alarming. The unexplained bruising, the constellations of red, pinpoint dots on her skin, the collapsing fatigue she had been experiencing — these were not indications of clumsiness or symptoms of a minor virus. Her life was in danger.
Shelton was prepped for a blood transfusion and for a bone marrow biopsy, but doctors already suspected what they’d find. She had acute lymphoblastic leukemia, or ALL, a blood cancer that rapidly becomes lethal.
“They came back in a few hours and said, ‘If you hadn’t come in, you would have been dead in a week,’” Shelton recalled. “The next thing I know, I’m on an ambulance to UVA in Charlottesville to get treatment.”
As Shelton’s head spun with her new reality, 200 miles away in Chapel Hill, UNC Professor of Medicine Dr. Jonathan Serody was on the phone with Baylor College of Medicine’s Dr. Malcolm Brenner, hatching a plan that one day would create the largest research program ever established at UNC’s Lineberger Comprehensive Cancer Research Center.
That plan also would become Shelton’s best chance at beating ALL.

Dr. Jonathan Serody
Serody, who’d investigated cellular-based vaccines to fight breast cancer, wanted Brenner to move his laboratory studies around a novel immunotherapy concept called chimeric antigen receptor T-cell therapy, or CAR-T, to Lineberger.
T-cells are the immune system’s warriors; they are skilled at protecting the body from obvious foreign invaders like viruses. But they can be guileless heroes when it comes to cancer.
Malignant cells are double agents, mutated versions of the body’s normal cells that can escape a T-cell’s detection. It’s never been a fair fight.
CAR-T aimed to change that. The idea involved removing a patient’s T-cells and customizing them like an Aston Martin in a James Bond movie. The T-cells are outfitted with unique receptors designed to recognize and bind to proteins, or antigens, found on the patient’s specific tumor cells. The souped-up cells are then multiplied in a lab and reintroduced to the patient’s blood through infusion.
When CAR-T cells enter the bloodstream, they’re poised to attack their targets, special agents on a mission.
Serody had been following CAR-T’s research for decades. In addition to completing an infectious disease fellowship at UNC in 1990, he’d been a bone marrow transplantation fellow in 1993 at Fred Hutchinson Cancer Center in Seattle, where some of the most powerful precursors to CAR-T had been established. For most of its progression, however, CAR-T had been considered such science fiction, so unlikely ever to work, that its researchers often were ridiculed by mainstream science.
“We were a minority of believers,” said Brenner, who had been investigating the science since the early 1990s. “Most people thought it was all nonsense.”
But by 2007, researchers were beginning to understand why early CAR-T lab models hadn’t clicked, and Serody wanted Lineberger to join the sci-fi club.
They’d have to do it without Brenner.
As much as he admired the research environment at Carolina, Brenner told Serody, no serious CAR-T scientist would move to an institution without a dedicated facility for building and multiplying the engineered cells. Brenner was talking about a hyper-meticulous space called a Good Manufacturing Practices facility, or GMP, that requires separate clean rooms — enclosed areas with tight restrictions on temperature, humidity and air filtration — for each CAR-T therapy target.
Fussy to build and expensive to maintain, GMPs are the bridge between CAR-T bench research and clinical trials; breakthroughs can’t happen without them. And meaningful CAR-T development also would require a team of clinical trial investigators and regulatory personnel to keep it on track. Few places boasted such a complicated infrastructure, and none were in the Southeast. In the U.S., the number of academic institutions that had the power to move their own CAR-T constructs from petri dish to mouse to manufacturing to patient was seven.
Starting from scratch, Serody was determined that Lineberger would be the eighth.
Dream team
In 2011 and 2012, CAR-T astounded its longtime detractors by saving lives in its first clinical trials. Three leukemia patients who had exhausted their treatment options — including a 7-year-old named Emily Whitehead who, like Shelton, had ALL — went into complete remission following CAR-T therapy at the University of Pennsylvania.
CAR-T still was not a home run; it was, and remains, a last-resort option because it can cause severe side effects — sometimes life-threatening — in the two weeks following infusion.
Whitehead almost died from the most commonly experienced problem, called cytokine release syndrome — a storm of inflammatory responses to the CAR-T battle within that can result in dangerously high fever and low blood pressure, as well as neurological toxicities. She was rescued with a medicine commonly given for rheumatoid arthritis.
In some patients, the cells just didn’t work. In others, the CAR-Ts extended lives but did not flourish long enough to prevent a relapse. And CAR-T so far was limited to blood cancers, excluding solid tumors that are responsible for most cancer deaths. The learning curve still was steep.
But CAR-T had cured an elementary schooler who had no hope, and her story launched a band of believers throughout science and the pharmaceutical industry. At Lineberger, after four years of hard lobbying by Serody — and after sending handfuls of North Carolinians elsewhere for CAR-T trials — leaders gave the green light to the new cancer assassins, allocating funds from the University Cancer Research Fund to build a GMP, recruit CAR-T researchers and chase the science forward.
Work on the $3.75 million, 6,000-square-foot GMP began in an existing building about four miles from campus in December 2014. Paul Eldridge, a Charlotte native who’d been running the Human Applications Laboratory at St. Jude Children’s Research Hospital in Memphis, joined Lineberger to direct it.
Eldridge was at the top in a niche field; he had had a hand in the early manufacturing of the CAR-T that had saved Whitehead, a construct that targeted an antigen known as CD19. His addition to the staff proved Lineberger was serious. That was useful when Carolina began wooing Dr. Gianpietro Dotti and Dr. Barbara Savoldo — husband and wife scientists in Brenner’s department who had contributed valuable science to CAR-T’s progression.
The two had reached a crossroads in their growth at Baylor and were planning to move home to Italy in 2013 when their phones began ringing with offers from across the U.S.

Dr. Jonathan Serody, left, had followed the research for decades and badly wanted Lineberger to be part of it. Dr. Gianpietro Dotti and Dr. Barbara Savoldo rounded out the dream team. (UNC)
Throughout academia and in Big Pharma, the race was on to jump in the CAR-T game. But Dotti and Savoldo had spent years listening to the research community scoff at their work, and their sudden popularity made them wary of trend-seekers who lacked commitment. They visited Lineberger four times over a year and a half before accepting its proposal.
“Everybody gives you the love and money and this and that,” said Dotti in a singsong cadence. “But we didn’t see the mentality that was needed to build the program anywhere else but UNC. Everybody offered us big stuff in the lab, but if we don’t do the next step — getting the work into patients — we were not interested.”
Beyond expectation
They were eager to get started. Lineberger’s first clinical trial opened in 2016, using crucial research Dotti and Savoldo had brought with them about a CAR-T target expressed on lymphoma cells known as CD30.
The results of Baylor’s first trial targeting CD30 had been underwhelming, with only one of nine patients achieving remission. Lymphoma presents challenges for CAR-T therapy that leukemia does not; for starters, its malignant cells are not swimming throughout the blood.
After the engineered cells enter the bloodstream, they must live long enough and travel far enough to reach the areas where cancerous lymph cells collect before they can mount an attack.
At Carolina, Serody suggested they give patients a chemotherapy regimen to knock out their normal immune cells before CAR-T infusion. The body then would be primed to recoup its losses, giving the CAR-Ts a fertile environment to multiply in greater numbers and reach their targets.
It was a winning strategy. More than 70 percent of patients in the trial — most of whom had Hodgkin lymphoma — responded to Lineberger’s CAR-T therapy. The trial’s final group of patients had the best results: Twelve of the last 16 achieved complete remission, and half of those have stayed cancer-free since. Some had significant disease and had never responded to other treatments; most had been through seven other failed therapies first.
“That’s just pretty spectacularly successful,” Serody said. On the strength of those findings, Lineberger won an $8 million grant from Stand Up to Cancer to collaborate on CAR-T for T-cell lymphoma.
The CD30 results have gained pharmaceutical company attention. But Savoldo isn’t content; she wants to increase that success rate and prevent relapse.
A new trial began in December 2018 that builds on the first, using a construct that Dotti and Savoldo began work on 10 years ago. In it, the CD30 CAR-T cell is supercharged further with a supply of GPS-like proteins that can read and track a signal relayed by lymphoma cells. That tweak should mean the CAR-Ts waste less time looking for their targets and live longer in the body, producing more — and more durable — remissions.
The first three patients have had impressive responses, even at the lowest possible CAR-T cell dose, Savoldo said.
The CAR-T process: (1) A patient’s T-cells are collected through a blood sample. (2) The cells are then modified to create chimeric antigen receptors (CAR), which help the T-cells target and destroy cancerous cells. (3) The CAR-T cells are multiplied, and millions of these attack cells are reintroduced into the patient’s body. (4) The CAR-T cells now can recognize and destroy cancerous cells. (Illustration by Haley Hodges ’19)
Lineberger’s CAR-T science has moved faster even than Serody anticipated. In November, there were nine active CAR-T clinical trials investigating lymphoma, leukemia and myeloma and at least two more coming soon. That’s prompted a GMP expansion that is expected to double the facility’s size by the end of 2020.
And while its CAR-T trials receive more patient referrals from North Carolina than any other state, more than half come from places like Texas and California. One came from Hawaii.
Serody, who manages the clinical side of CAR-T work as well as his own immunology research lab, barely has time to look away from the hive of monitors that encircle his standing desk, let alone break down the journey that has made UNC, in four short years, a burgeoning headquarters for hope.
“If you want to try to investigate new diseases, new targets, new opportunities in CAR-T,” Serody said, “we’re the only place that does that in this region. We’re starting to outgrow the little mom-and-pop store we were four years ago.”
Hope, hype and skepticism
Ask Baylor’s Malcolm Brenner if he’s gratified that CAR-T — which won its first two FDA approvals in 2017 — is finally sitting at the grown-up table, and he gives a nervous laugh.
“I’m a little bit worried about the hype,” he said. “We’ve got a long haul ahead.”
CAR-T is still largely an experimental therapy — a futuristic, staggering, frustrating therapy that sometimes works and sometimes does not. As miraculous as they are, Kymriah and Yescarta, the commercial CAR-T products that treat ALL and large B-cell lymphoma, are what Dotti calls first-generation. They could be obsolete in a few years because of the pace of research to improve upon them.
CAR-T is still largely experimental — a futuristic, staggering, frustrating therapy that sometimes works and sometimes does not. As miraculous as they are, the commercial CAR-T products could be obsolete in a few years because of the pace of research to improve upon them.
And for some multiple relapsed patients, like Sabrina Shelton, they remain out of reach.
After a complete remission with chemotherapy in 2007, Shelton’s cancer returned in 2012, in 2015 and for a third time last fall. Even during those too-brief stretches when leukemia finally seemed to have left Shelton’s body, it would not leave her mind; it loomed over all her decision-making for 11 years. Shelton avoided applying for jobs that were too far from her oncologist. She asked for more frequent checkups, even when doctors suggested fewer. She put off traveling for fun.
“I just honestly want to be done with treatments,” said Shelton, who earned a master’s degree in human services counseling in 2016 and volunteers at a YWCA bridal thrift store that benefits victims of domestic violence. “I just want to go live where I want to live without hesitation.”
CAR-T was where Shelton was headed next. But due to the severe toxicity it could cause, Kymriah had been approved for ALL patients only up to age 25, whose less-battered immune systems could better withstand its often-wild effects. Shelton was now 33.
Enter Lineberger’s CD19 CAR-T trial, which targets the same antigen as Kymriah but enrolls adult patients. It also features a novel twist: Dotti built the CAR-Ts with a stop switch he helped develop at Baylor, a mechanism that can turn off CAR-T cells if a patient begins to experience life-threatening cytokine release syndrome after infusion.
The switch helps set Lineberger apart in safety, which has been a growing concern as immunotherapy has gained steam; in 2017, the FDA halted a commercial CD19 CAR-T trial after seven adult participants died from toxicity.
Still, the unknowns bothered Shelton, who was told she would be the fifth patient treated in the trial. When she asked how long the infused CAR-T cells would work, Lineberger clinicians could not tell her — no physician could have. Despite its exceptional science and engineering, CAR-T is still a leap of faith for all involved.
“Everyone in the field has a healthy skepticism about these cells,” said Dr. Matthew Foster ’97 (’02 MD), an associate professor of medicine at UNC and the principal investigator in its CD19 study. “We do know that when they do not persist [in the blood], the patient is at risk for relapse. Whether they need to persist for six months, one year, five years or longer is uncertain. We are learning as we go.”
Patient One in UNC’s CD19 trial was a young man with a grave prognosis who’d never achieved remission until CAR-T. When they got the news that he was disease-free, Serody and Lineberger Director Dr. H. Shelton Earp ’70 (MD, ’72 MS) high-fived.
“That was a really, really good day,” Serody said. “But you don’t sit in the moment forever. For what all of us do, the majority of days, things don’t work.”
For every spectacular result in a CAR-T CD30 Hodgkin lymphoma patient, Serody and company have multiple concerns to address in others: Why did Patient One in the ALL trial relapse months later? Why has the myeloma study yet to see a remission among its four participants? Should they adjust the dosage, tinker with the construct, get the patients into trial sooner?
The good days don’t happen all the time, Serody said, but their echoes remind researchers that the challenges are solvable.
After her infusion in 2019, Shelton spent a week in the ICU with a high fever — a minor problem on the spectrum of possibilities, which was managed without the stop switch. CAR-T therapy, she said, was less taxing than any leukemia treatment she’s had in the past 11 years.
But CAR-T has a long way to go before it could replace chemo as a first-line treatment — and it may never get there. Many patients owe their lives to surgery and chemotherapy, Dotti said, because those methods remain the best way to eradicate large amounts of tumor quickly. What CAR-T’s “live drugs” can and should do, he said, is work in combination with chemo to maintain or extend remissions, providing a one-two punch for a better quality of life with fewer long-term side effects.
CAR-T also is more effective when it’s unleashed on a less battered immune system; currently, patients have had four or more standard therapies before they become eligible.
“For sure, it’s good to move CAR-T earlier in treatment,” Dotti said. “I don’t have any doubt about that.”
Custom-Made Therapy Comes With Sticker Shock
What’s the difference between commercial CAR-T therapy and the cancer treatments that have come before it? The same things, said Lineberger researcher Dr. Gianpietro Dotti, that separate fine dining from a mass-produced meal in a box.
Personalization … and price.
“You have a restaurant, and every day you make a separate meal for each guest,” he said. “With CAR-T, it’s the same. You make the cells for a specific person. It’s a live product that you need to make all the time.”
The sticker prices for a single infusion of Kymriah ($475,000) and Yescarta ($373,000), which treat blood cancers and are the only FDA-approved CAR-T therapies on the market, have raised caution flags about the treatment’s sustainability. A 2019 Reuters report described how hospitals that still lack coverage agreements with insurers for commercial CAR-Ts sometimes refer their lymphoma patients to clinical trials instead.
And as researchers at UNC and elsewhere discover CAR-T therapies that can defeat more common cancers of the lung, prostate and breast, that price point — which does not include follow-up care — would burst an already swollen health care system, with Medicare and insurance companies scrambling to cover CAR-T for more patients.
“We have to get through the science first,” said UNC Lineberger Comprehensive Cancer Center chief Dr. Shelley Earp ’70 (MD, ’72 MS). But when more cancers become treatable with CAR-T, he said, UNC won’t be idle as the market inches toward a sustainable model. He envisions Lineberger becoming a CAR-T treatment center for all patients, not just those on clinical trials.
“Commercialization would be desirable across the country, but that may take a long time in solid tumors,” Earp said. “If we’re successful in doing this, we want to be able to scale it so that we have a larger capacity to treat people here at UNC.”
— Beth McNichol ’95
At her three-month checkup, Shelton’s special brew of CAR-Ts was still doing its thing.
She was in remission.
“It’s still scary to know that it could only last for short-term,” said Shelton, whose CAR-T cell activity will be checked every three months, “but at least I’m grateful for the outcome that it’s had so far. It’s been a long road.”
In the lobby of the N.C. Cancer Hospital, Shelton talked about the dreams she’d chase if ALL would finally leave her alone: more travel, less fear and a career helping other young cancer victims. She paused for a long beat, searching for words to describe her health now.
“I think it feels normal,” she said, looking out of the window as patients were wheeled to their cars. She turned back and smiled gently, two dimples forming parentheses around her mouth. “I can’t completely remember what normal feels like. But I feel like I’m there.”
‘The guts to believe’
From the outset, Lineberger has sought to push the boundaries of discovery where commercial biotech, bound by stockholders, cannot. The challenge for the next 10 years is to unleash the therapy on solid tumors, which develop their own hostile microenvironments full of immuno-subterfuge.
Last summer, UNC’s CAR-T program entered the solid tumor frontier when it began treating children for neuroblastoma, a rare pediatric cancer with a painful progression and a grim prognosis. In February 2019, Dotti identified a new target for glioblastoma, the deadliest type of brain cancer. His novel CAR-T construct to fight it is in production and testing at the GMP and will be tried in patients beginning in early 2020, as will another target for ovarian cancer.
Achieving dramatic CAR-T responses in any of these diseases would represent an Armstrong-like step for mankind in the fight against cancer. For Assistant Professor of Medicine Dr. George Hucks, a pediatric oncologist and the principal investigator in UNC’s neuroblastoma study, those leaps can’t come fast enough.
“Recently we had a person who all she wanted to do was go to her prom and graduation,” Hucks said. “Unfortunately, the type of cancer she has does not have a CAR-T option right now. To be able to have this completely new modality for someone like that, who’s tried everything else … who just wants to go to her prom? The phrase ‘life-saving’ doesn’t really catch the spirit of what that would mean.”
Now 15, Emily Whitehead, the best-known CAR-T hero, is not just a teenager who survived her unbeatable cancer; she’s a reminder that all the best ideas science has ever dreamed of once were dismissed as nonsense. In that respect, CAR-T researchers share an important trait with their patients: perseverance. It’s what Earp called “the guts to believe.”
“My mother has what I call a regular job,” Savoldo said. “She used to ask me, ‘Why do you work so late? Why don’t you get someone else to do it?’ I told her, ‘Because I want to do it! Because I need to see the end of it.’
“In this field,” she said, “you could create something magical.” Like an armada of futuristic T-cells, charging through the bloodstream in search of whatever normal feels like, in search of a cure.
Beth McNichol ’95, a freelance writer based in Raleigh, is a former associate editor for the Review.
Thanks for reading the Carolina Alumni Review
Carolina Alumni members, sign in to continue reading.
Not yet a member? Become one today.